Not only that, let us think about these: Why does pollen grains move in water? Why does the food colouring spread out in a bucket of water? All these will be explained in this post.
Firstly, let us state the simple knowledge everyone should know.
Matter is anything that has mass and occupies space.
All matter can exist in 3 physical states: solid, liquid & gas, depending on the temperature and pressure of their surroundings.
What are the differences among solids, liquids and gases?
- A solid has a fixed shape and a fixed volume. It is not compressible.
- A liquid has a fixed volume but it does not have a fixed shape. It can flow and takes the shape of its container. It is not compressible.
- A gas has no shape, no surface and no fixed volume. It is highly compressible.
Liquid
| |
Diagram
|
|
Arrangement
|
|
Movement
|
|
Gas
| |
Diagram
|
|
Arrangement
|
|
Movement
|
|
That is how, in detail, we classify matter. Now let us go to the complicated topics. What is the Kinetic Particle Theory?
The Kinetic Particle Theory states that matter is made of a large number of tiny particles (atoms or molecules), which are in continuous and random motion.
The Kinetic Particle Theory could be proven by Brownian Motion and Diffusion.
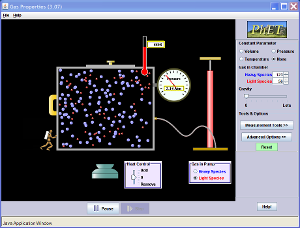
Brownian Motion, is the continuous and random motion of small solid particles in fluids (liquids and gases). Brownian motion had also proved the existence of particles that cannot be observed with a normal microscope.
Brownian Motion can be observed by placing smoke particles in air. The smoke particles, when seen under the microscope, can be seen to be moving. If heat is supplied, the motion of the smoke particles become more vigorous. Brownian Motion can also be observed by placing pollen grains in water. The pollen grains's movement is caused by the bombardment by the water molecules.
Below is an Java applet. Using small particles as the particles of a gas or liquid, and a big particles as a small solid, the Brownian Motion can be shown. Also, watch the effect when the heat has been changed.
Diffusion shows that particles move randomly from a region of high concentration to lower concentration.
If a bottle of perfume is opened in one
corner of a room, we can smell it in another corner after a very short time as the perfume molecules have traveled from the bottle to your nose
through the air. This spreading of molecules is called diffusion.
Bromine vapour can be used to show
diffusion of gases. Bromine is a red brown liquid at room temperature. When it evaporates, it becomes a brown vapour. When a little bromine vapour is
released into a vacuum, the brown vapour spreads through the vacuum almost at
once, showing that the bromine molecules are moving at very high speed.
 |
| Diffusion of Bromine |
If bromine
vapour is released into a similar space full of air, the brown vapour still
spreads quickly through the space but very much slower than in a vacuum. This
is because the bromine molecules keep hitting the air molecules which get in
the way. The air acts as a resistance.
The
rate of diffusion of gases depends on the temperature and the density of the
gases. The higher the temperature, the faster the diffusion, but the greater
the density of the molecules, the slower the diffusion. Gases can also
diffuse through walls which have pores slightly bigger than the size of the gas
molecule.
Diffusion also takes place in
liquids, though at a very much slower rate. An unstirred cup of
coffee with milk will become uniformly coloured after many
hours. In the school laboratory, diffusion of liquids can be shown using copper
(II) sulfate solution and water as shown in the figure below. The two layers
become uniformly mixed after a while.
 |
| Diffusion of Liquids |




No comments:
Post a Comment